SHOCKER: A list of Killer Drugs that were FDA approved
We THOUGHT that “FDA Approved” meant something … turns out, it’s a bribery game
BS”D
If you had Alzheimer’s, would you want to receive a drug that would likely cause you brain swelling and bleeding?
Or would you prefer a different dementia drug, the one prone to causing stomach bleeding and heart problems?
If you were being treated for cancer, would you want drugs that show proven benefit in extending lifespan - or ones that will just make you feel sick with no gain to show for it?
Yes, all the above “medicines” actually exist.
If you were a cancer survivor, would you want to be bullied into taking a drug “to prevent recurrence” which had a 3.8% chance of killing you outright, up to 10% chance of liver toxicity, a sizable chance of giving you a life-threatening infection, and up to 10% chance of giving you a heart attack … and not even have these risks explained to you?
These are all real-life scenarios that really happened. Don’t worry, the drugs were all FDA approved, so they’re safe!
This article will highlight some of the shameful excuses for medicines which are making it to market under the “watchful eye” of the FDA (Fraud and Death Association.)
Dementia Drugs
The most unbelievable example of a dangerous and useless drug that I can think of is Aduhelm, a $56,000-a-year Alzheimer’s drug which was FDA fast-track approved in June 2021, and pulled from the market in January 2024, because it didn’t do anything except cause brain swelling, brain bleeds, and other horrible side effects. In fact, brain swelling or brain bleeding was found in 41% of patients enrolled in its studies.
How did such a disastrous drug come to be created? A Midwestern Doctor explains:
The existing dogma within Alzheimer's research is that Alzheimer's disease results from the buildup of amyloid plaques within the brain which then cause brain damage that leads to Alzheimer's disease. The majority of research for treating Alzheimer's disease has thus been targeted at eliminating these plaques.
But they are barking up the wrong tree.
From Science.org:
Hundreds of clinical trials of amyloid-targeted therapies have yielded few glimmers of promise, however; only the underwhelming Aduhelm has gained FDA approval. Yet Aβ [amyloid-beta] still dominates research and drug development. NIH spent about $1.6 billion on projects that mention amyloids in this fiscal year, about half its overall Alzheimer’s funding. Scientists who advance other potential Alzheimer’s causes, such as immune dysfunction or inflammation, complain they have been sidelined by the “amyloid mafia.”
Neurology Advisor explains:
The FDA approval board assumed that the reduction of amyloid plaques on a positron emission tomography (PET) scan was a surrogate endpoint for AD, despite the inability to demonstrate any meaningful clinical benefit of that observed change.4
In other words, less amyloid plaques seen on PET scan results were absolutely meaningless - just a made-up goal - because the patients didn’t have any improvement in their Alzheimer’s. Yet the drug company and the FDA kept pretending they were somehow helping.
In 2016, a team of researchers published phase 1b trials demonstrating aducanumab’s ability to lower amyloid plaque levels in patients’ brains. Two phase III trials (ENGAGE and EMERGE; ClinicalTrials.gov Identifiers: NCT02477800 and NCT02484547, respectively) included participants with early-stage AD. However, both of these trials were discontinued in 2019 after a futility analysis.
Unbelievably, though:
The FDA … suggested Biogen [the manufacturer] file for approval to market the drug, which was granted in June 2021 against expert recommendations.4
Ultimately, aducanumab was widely criticized for lackluster evidence and harrowing side effects. Some of the more serious drug-related adverse events noted in clinical trials included brain edema, microhemorrhages, and superficial siderosis. Dizziness, falls, hallucinations, and vision changes were some of the more frequently reported side effects.4 In addition, when aducanumab trials failed to demonstrate improvements in cognitive test scores, the FDA accepted reduced amyloid levels as a revised surrogate endpoint, effectively making an assumption that was yet to be supported with data.1
Medications for AD are not typically considered appropriate for the accelerated approval tract, so this decision was met with strong opposition. In fact, the FDA’s own expert advisory panel voted 10 to 0 (with 1 abstention) to reject the accelerated marketing approval. After it was granted anyway, 3 advisors on the panel resigned.1
Why in the world would the FDA have approved a useless and dangerous drug which its own expert panel voted 10 to 0 against? Did Biogen bribe the FDA to approve it?
A Midwestern Doctor: There … appears to have been inappropriate relationships within the FDA that led to Aduhelm receiving an accelerated approval.
Note: Patients were still able to obtain this horrific drug until last month.
Here is an older dementia drug, Donepezil (Aricept.) When someone I know was discharged from the ER last spring with this drug on their medication list, I thought I’d better look it up.
Indeed, from Cleveland Clinic’s website page about Donepezil:
What side effects may I notice from receiving this medication?
Side effects that you should report to your care team as soon as possible:
Allergic reactions—skin rash, itching, hives, swelling of the face, lips, tongue, or throat
Peptic ulcer—burning stomach pain, loss of appetite, bloating, burping, heartburn, nausea, vomiting
Seizures
Slow heartbeat—dizziness, feeling faint or lightheaded, confusion, trouble breathing, unusual weakness or fatigue
Stomach bleeding—bloody or black, tar-like stools, vomiting blood or brown material that looks like coffee grounds
Trouble passing urine.
(End quotes from website.)
Are patients or family informed that the medicine which might somewhat aid their memory may also cause seizures, trouble breathing, dizziness (which could lead to falls), bloody stools, or confusion?
Pharmacy Collection
•I know someone who was severely injured, to the point of disability, by the OTC hair loss drug Minoxidil. He sustained chromosomal damage and unfortunately has been turned away at every hospital at which he has tried to obtain help for his numerous resulting health problems. His research uncovered that he is far from alone in being harmed by Minoxidil.
There are, of course, innumerable other drug disasters which are hurting and killing so many of the elderly and most vulnerable: SSRIs, statins, aggressively prescribed blood pressure medications, and much more.
To quote A Midwestern Doctor, “frequently, when you dig into medical myths, you discover that many of the dogmas that underlie a popular drug are actually sales slogans a marketing company created. For instance:
•Cholesterol lowering statins are widely prescribed despite the fact lowering cholesterol does not prevent heart disease (in fact cholesterol protects you, so when it’s low, you more likely to die), statins don’t prevent death, and these drugs harm 20% of users (often severely).” Look up The Great Statin Scam.
More from AMD:
“Sadly, statins are not the only mass-prescribed drug that’s marketed on deceptive premises and frequently makes the problem it “treats” worse. For example:
•SSRIs: A chemical imbalance from low serotonin was never linked to depression (in fact patients who commit suicide are found to have elevated brain serotonin).
•Antacids: Acid reflux is due to too little acid in the stomach (as acidity gives the stomach’s opening the signal to close). However, in medical school, we are always taught it is due to too much acidity.
•"Sleeping" pills are actually sedatives that block the restorative phase of the sleep cycle.
Each of these drugs is immensely harmful to their users, but due to how effectively their myths were established (just like “safe and effective”) they continue to be used by large numbers of people and harm them.”
•Here, A Midwestern Doctor explains the dangers of blood pressure medications and especially of excessively lowering blood pressure in elderly individuals: https://www.midwesterndoctor.com/p/the-great-dangers-of-blood-pressure
Also, AMD:
•NSAIDS kill tens of thousands of Americans each year and seriously injure far more.
•“To create a new market, NSAIDs like Vioxx were created that … had a huge stroke and heart attack risk. However, despite evidence of harm continuing to mount, Merck and the FDA kept on covering it up, and when the drug was finally pulled, Vioxx was estimated to have killed 120,000 people. Sadly, as the FDA scientist who fought to get it off the market testified, this is business as usual.” (End of quotes by AMD.)
•Robert Yoho MD elaborated on the severe harms and deaths not infrequently caused by Tylenol, in this article: https://robertyoho.substack.com/p/320-tylenol-is-a-pharma-atrocity
Excerpt:
“Bayer introduced phenacetin (BW: the predecessor to Tylenol, and its precursor in the body) in 1887. It was born out of the need to dispose of 30,000 pounds of para nitro-phenol, a substance Bayer used for dyes. They marketed it into a global success.
“Phenacetin studies on animals uncovered multiple side effects, including cancer and renal disease. It was learned in 1948 that the body converts phenacetin into APAP (Tylenol), which was considered at the time too toxic to market. It took another 25 years for Canada to ban phenacetin (1973) and 35 years for the U.S. to do the same. APAP should also have been banned, but instead, it remained over-the-counter.
“Tylenol is the leading cause of drug death from liver failure. Taking just four grams (eight 500 mg capsules) of Tylenol in 24 hours may cause slow, agonizing fatal liver necrosis. When combined with alcohol, this may occur after only two grams (four pills). For survival, treatment must begin within about eight hours.
“Acetaminophen toxicity is the second most common cause of liver transplantation worldwide and the most common cause of liver transplantation in the US. It is responsible for 56,000 emergency department visits, 2,600 hospitalizations, and 500 deaths per year in the United States.” (Other sources: 1000 deaths).
BW: This was so disturbing that I had to double check it myself. Robert Yoho is telling the truth! Google helpfully explained:
Phenacetin was withdrawn from the U.S. market in 1983 due to its association with kidney disease and potential risks of tumorigenicity. It was also withdrawn from use in Canada in 1973.
Toxicity
Phenacetin is harmful if swallowed or inhaled, and can cause kidney, liver, and blood disorders. The IARC Working Group classifies phenacetin as a group 2A substance, which means it is reasonably anticipated to be a human carcinogen.
And: “Yes, Phenacetin is considered a precursor to Tylenol (acetaminophen) because when metabolized in the body, phenacetin breaks down into acetaminophen as its primary metabolite, meaning that Tylenol is essentially the active ingredient produced from phenacetin when it is processed by the body; hence, phenacetin acts as a precursor to acetaminophen (Tylenol).”
Back to Robert Yoho MD:
“A long-term study found a 63% increase in the death rate in regular users of APAP. “There is a consistent association of increased mortality as well as cardiovascular (stroke, myocardial infarction), gastrointestinal (ulcers, bleeding) and renal adverse effects with taking higher dose of paracetamol… It is now the most commonly used analgesic in the world” (from Wikipedia 2011 and Jan 2024).
BW: Remember, APAP is Tylenol.
“APAP is a neurotoxin. A study of the elderly found the proportion with dementia who used APAP was 50.6% compared to 21.4% who did not take it. Alzheimer’s is a pandemic, and may be related to this.
“A study of 2,919 Norwegian mothers looked at Tylenol use during two of their pregnancies, one with the drug and another without it. To reduce confounding familial and genetic factors, same-sex siblings were compared. The mothers who used APAP for 28 days or longer during pregnancy had offspring whose cognitive function was statistically lower than their siblings. Other studies confirmed this. Studies on pregnant rats are further support for APAP’s neurotoxicity.
“Human studies show that APAP increases diabetes by 20 percent or more in long-term users. At therapeutic dosages, acetaminophen can be kidney toxic for patients who are glutathione-depleted or who are taking drugs that stimulate liver P-450 microsomal oxidase enzymes.
“APAP causes mitochondrial dysfunction: “These findings indicate that paracetamol toxicity results in an impairment of mitochondrial function which precedes the loss of blood membrane integrity.”
(End of quotes by Robert Yoho MD.)
➡️No wonder a study found that reducing the amount of medications an elderly person takes reduces their risk of death: https://pubmed.ncbi.nlm.nih.gov/17642388/
Study excerpt:
Results: A total of 332 different drugs were discontinued in 119 patients (average of 2.8 drugs per patient) and was not associated with significant adverse effects. The overall rate of drug discontinuation failure was 18% of all patients and 10% of all drugs. The 1 year mortality rate was 45% in the control group but only 21% in the study group (P < 0.001, chi-square test). The patients' annual referral rate to acute care facilities was 30% in the control group but only 11.8% in the study group (P < 0.002). The intervention was associated with a substantial decrease in the cost of drugs.
Conclusions: Application of the geriatric-palliative methodology in the disabled elderly enables simultaneous discontinuation of several medications and yields a number of benefits: reduction in mortality rates and referrals to acute care facilities, lower costs, and improved quality of living.
Disclaimer: Please do not use this article as medical advice in any way. Seek personal guidance from an educated medical practitioner you trust.
Cancer Treatments
In July 2023, a study conducted in Sweden and published by Springer Nature shockingly found that out of 22 cancer drugs investigated, only 7 actually showed proven benefits in extending lifespan or improving quality of life.
This means, as substack author Ben Fen explains, that 2 out of 3 cancer drugs in widespread use show little to no benefit to quality of life or longevity.
https://link.springer.com/article/10.1007/s40261-023-01285-4
Ben Fen elaborates on the Swedish study:
“Scientists at the University of Gothenburg in Sweden found that approximately two out of three cancer drugs prove to be useless for patients after examining the eventual outcome on patients in terms of longevity and quality of life measures.
“The details of the study are in the original article referenced below but here are the highlights. The Swedish team examined 22 cancer drugs approved for use in Sweden over the last 10 years, examining other studies that tested these drugs’ ability to improve quality of life or extend lifespan. The names of the specific drugs studied are listed below. These studies followed the drug’s performance for an average of 6.6 years after the drug was released for use.
Cancer Drugs studied in Chauca Strand et al., (2023)
Everolimus
Nilotinib®
Axitinib
Brentuximab or Vedotin
BosutinibDabrafenib
CabozantinibOlaparib
Idelalisib
Ponatinib 1
Ponatinib 2
Ceretinib
PalbociclibOsimertinib
Alectinib 1
Fulvestrant
Ribociclib
Alectinib 2Lorlatinib
Niraparib
Venetoclax
Larotrectinib
“They found that only seven of the 22 drugs had at least one study which showed a clear benefit for cancer patients. Randomized controlled trials on the other 15 failed to show any measurable benefits for those with cancer. Only one, one!, out of the 22 drugs in the study showed an ability to both improve the quality of life and extend lifespans for patients. From Strand et al. (2023), “one drug, osimertinib, was found to improve both OS (longevity) and QoL (quality of life) for the indication of advanced NSCLC with EGFR T790M mutations. The improvements in median OS for these ranged from 2 to 13 months (n = 5).”
83% of the cancer drugs tested (18 out of the 22) did not
significantly increase or decrease longevity or quality of life.
“A deep dive into the meat of the paper indicates that the situation regarding the efficacy of these cancer drugs is even worse than the above headline reveals. Rather than the majority of the drugs simply being useless, some of them actually make things worse with respect to longevity and quality of life! Two drugs, axitinib and “everolimus, the pooled effect estimate showed a significant negative impact on OS (longevity)”. Furthermore, the authors state that 83% or 18 of the 22 drugs tested did not significantly increase or decrease longevity or quality of life. The authors did not indicate the direction of the trend. We can only speculate as to whether the trend was toward helping the patient or hurting the patient.”
End of quotes from Ben Fen, see his article here: https://fenbendazole.substack.com/p/swedish-medical-report-traditional
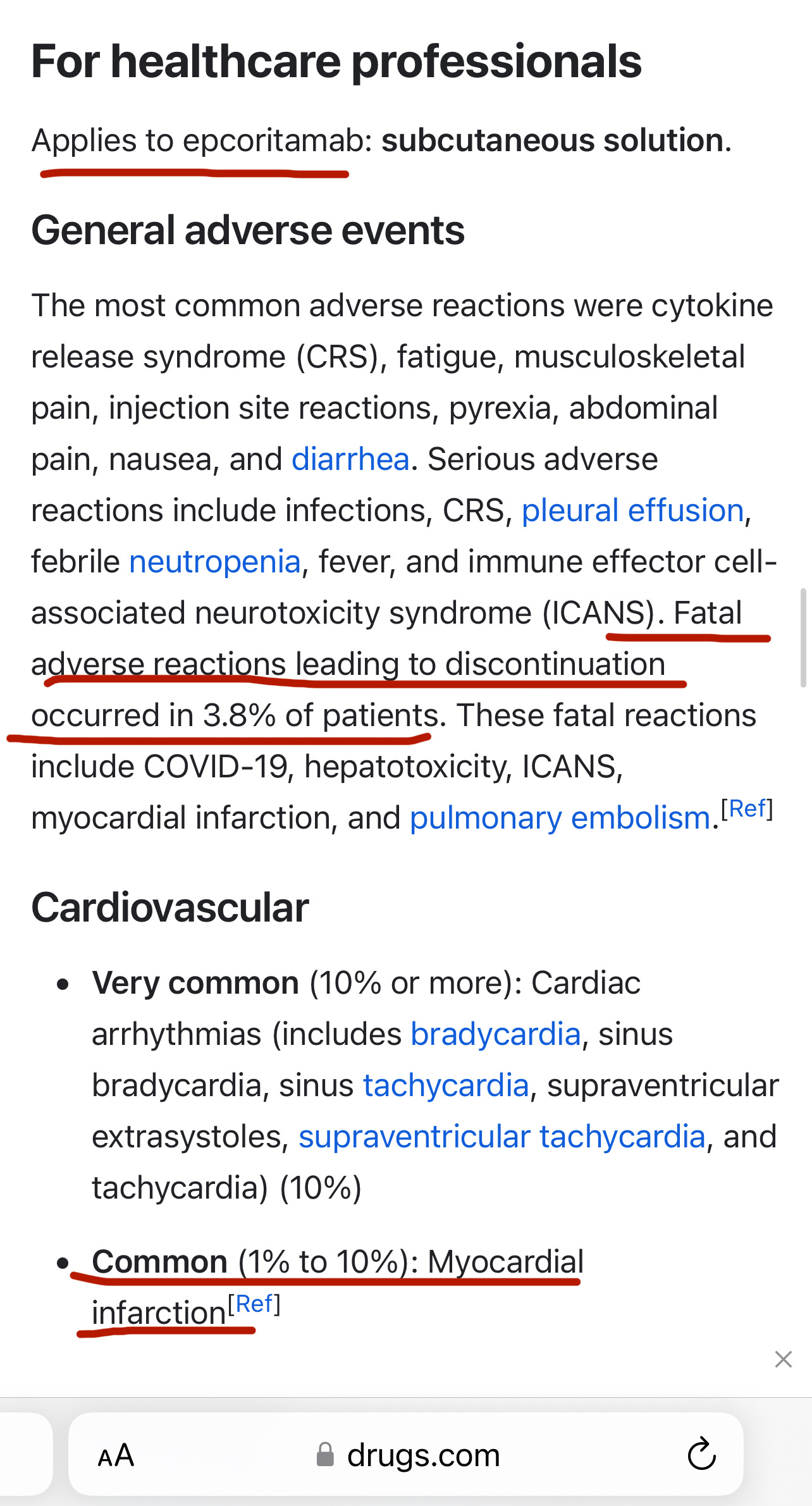
Epkinly (Epcoritamab)
I assume this drug has helped save some DLBCL cancer patients, even as it killed others. But when a woman I was talking to told me how she was just about forced to take Epkinly, after she was already well, “to keep the cancer from coming back,” and then was having very worrying symptoms, I looked the drug up, and I was horrified:
https://www.epkinlyhcp.com/clinical-trial-results
Yes, you saw correctly. Epkinly killed almost 4 out of 100 people who took it. It gave up to 10 in 100 people a heart attack, and gave up to 40% of patients in one trial serious infections (with 6% in that trial dying from the infections.) But it’s FDA approved!
Do you think my new friend had at least gotten an honest face-to-face discussion from her doctor about what the very possible adverse effects of the drug are - vs. the risks of the cancer recurring, and what other prophylactic treatments might possibly be used instead - so she could have constructive conversations with her loved ones and reach an informed decision? Or that she might at least know of danger signals, like signs of heart attack or pleural effusion, to be on the alert for? No.
Bottom line: If your loved one is prescribed any drug, don’t just trust. ASK QUESTIONS!
•What are the risks that this drug can cause death or serious side effects?
•What are the benefits of this drug and how likely am I to actually reap these benefits?
•Is the drug absolutely necessary or can it be foregone or substituted with something safer?
Your doctor may not know the answers - so try researching yourself and discussing your findings with him.
Please seek knowledgeable guidance, and do not make any medical decisions based on this article.
May G-d open the eyes of everyone to the truth.







Not surprised one bit.. You may want to do a report on what products were not approved that were beneficial instead they approved the ones that were deadly and more costly to the Public, but bought in more money for pHARMA and also what they recalled that had benefits for dis ease, especially covid19. Obviously what they approve is no good and what they do not approve or recall is actually good for you, that is how the FDA=Fatal Drugs Approved operates now.. They all should have their bank accounts checked..
Thanks for reporting.