The fight for SAFE BLOOD and for a RETURN to LOGICAL thought and true science
Inside a Court Case: COVID-19 Jabs and Blood Transfusion Products
BS”D
Many of you followed the story of Baby Will in New Zealand, whose parents sought to have him receive safe blood from private unvaccinated donors for his heart surgery. The blood was donated, but the hospital/doctors refused, took the parents to court, and took custody of the baby for the duration of the surgery.
It is suspected that the hospital ended up quietly using the unvaxxed blood anyway, because they feared complications in this case which had been publicized worldwide. As of the last I heard, thank G-d the baby is OK.
But the documents submitted in the court case are fascinating. The affidavits submitted by both sides (I read two of them, the first and the last) provide an astonishing contrast between a case made by emotion, with no scientific evidence provided, (the New Zealand hospital doctor arguing against the parents’ wishes) and a case thoroughly grounded in scientific evidence (Dr. Byram Bridle, arguing for the unvaxxed private blood donation.)
But guess how the judge ruled?
See Dr. Byram Bridle’s article and please be sure to see affidavits #1 and #6.
Inside a Court Case
COVID-19 Jabs and Blood Transfusion Products
There are a growing number of debates occurring around the world with respect to COVID-19 ‘vaccines’ and the safety of blood products being used in medical procedures. This is largely being driven by rapidly accumulating evidence in two areas. First, there are now plenty of publications showing that public guarantees that injectable COVID-19 products do not get widely distributed throughout the body were false. Secondly, there is a large body of literature showing that components and derivatives of the COVID-19 ‘vaccines’ have many potential mechanisms of harm should these products get beyond the injection site and local draining lymph nodes.
I was asked to serve the High Court of New Zealand as an expert witness in a case about a baby (Baby W) who required life-saving surgery for a heart condition.
The parents requested that the surgeons utilize blood from donors that had not received a COVID-19 jab. Their rationale was to ensure that there would be no risk of potentially harmful contaminants from COVID-19 inoculations being introduced into the circulation of the infant.
The core issue that I was asked to address is whether there was the potential for Pfizer’s COVID-19 ‘vaccine’, and/or components (i.e., lipid nanoparticles, synthetic mRNA) and/or derivatives thereof (i.e., spike protein) to be in the blood of people who donated following receiving a jab. I was also to note whether any of these contaminants would have the potential to cause harm should they be introduced into the systemic circulation of an infant.
At short notice, I submitted an affidavit to assist the court with rendering a decision. In addition to this, five affidavits were provided, at short notice, by three physicians. Only two of these were available at the time that I wrote mine.
The court’s decision went against the parents. As a result, joint custody was awarded to a physician to ensure the surgery could be performed as they saw fit, with the baby being returned to the parents afterwards. The joint custody is to remain in place until the final post-surgical check-up.
Redacted copies of all six affidavits have been provided to me. Since they are part of the public record I thought you might like to take a look at them. I have provided links to them below.
In the setting of a court, one is to review all affidavits and determine where the overall weight of the scientific evidence lies. Part of this process is to evaluate the experts that are providing the evidence, as well as the volume and quality of the evidence.
When reviewing these affidavits I encourage you to ask yourself the following:
How many published peer-reviewed scientific papers are cited in the affidavit? Ideally, there should be quite a few. Otherwise, how can the affidavit be objective and scientifically accurate?
How much raw data are presented and overlaid with interpretations? An ability to do this demonstrates genuine expertise.
Overall, who demonstrates the greatest mechanistic understanding of the science?
I also encourage readers to avoid getting distracted by hearsay evidence such as deferrals to statements made by organizations. This is what I like to refer to as ‘reputational science’. Many statements made by public health and health regulatory agencies are all-too-often of such low scientific rigor that they would never pass the bar required for consideration in a peer-reviewed journal. Among many other things, authors are typically not disclosed, nor are potential conflicts of interest, citations are often absent, few in number, or highly selective, materials and methods are often not described, etc. Many people who have not followed the publication of original scientific findings and/or who do not understand it at the most fundamental level often lean heavily on what various organizations have to say when composing affidavits.
Further, a true expert is not swayed just by the conclusions drawn by authors of papers. Instead, they look at the original raw data on which any conclusions rely. Of even greater importance, an expert should always look at the methods and materials used to generate the data to determine if they were appropriate. Conclusions are not valid if the data and/or methods used to produce them are invalid.
Another consideration is that one should never rely on the number of people drawing a particular conclusion. Scientific truths should never be defined by a majority vote. It is the overall weight of validated data, adjusted for its quality, that defines scientific paradigms.
Finally, when it comes to medical risk-benefit analyses, one must always consider what data are missing. Are there key questions that need to be answered? It is never appropriate to assume safety, especially in the absence of active safety monitoring programs being performed in the context of rigorously controlled clinical trials. The formerly cherished precautionary principle advises that people promoting a new medical product should prove safety rather than the onus being placed on bystander scientists to prove harm.
Here are links to the six affidavits…
Link to Dr. Bridle’s original article:
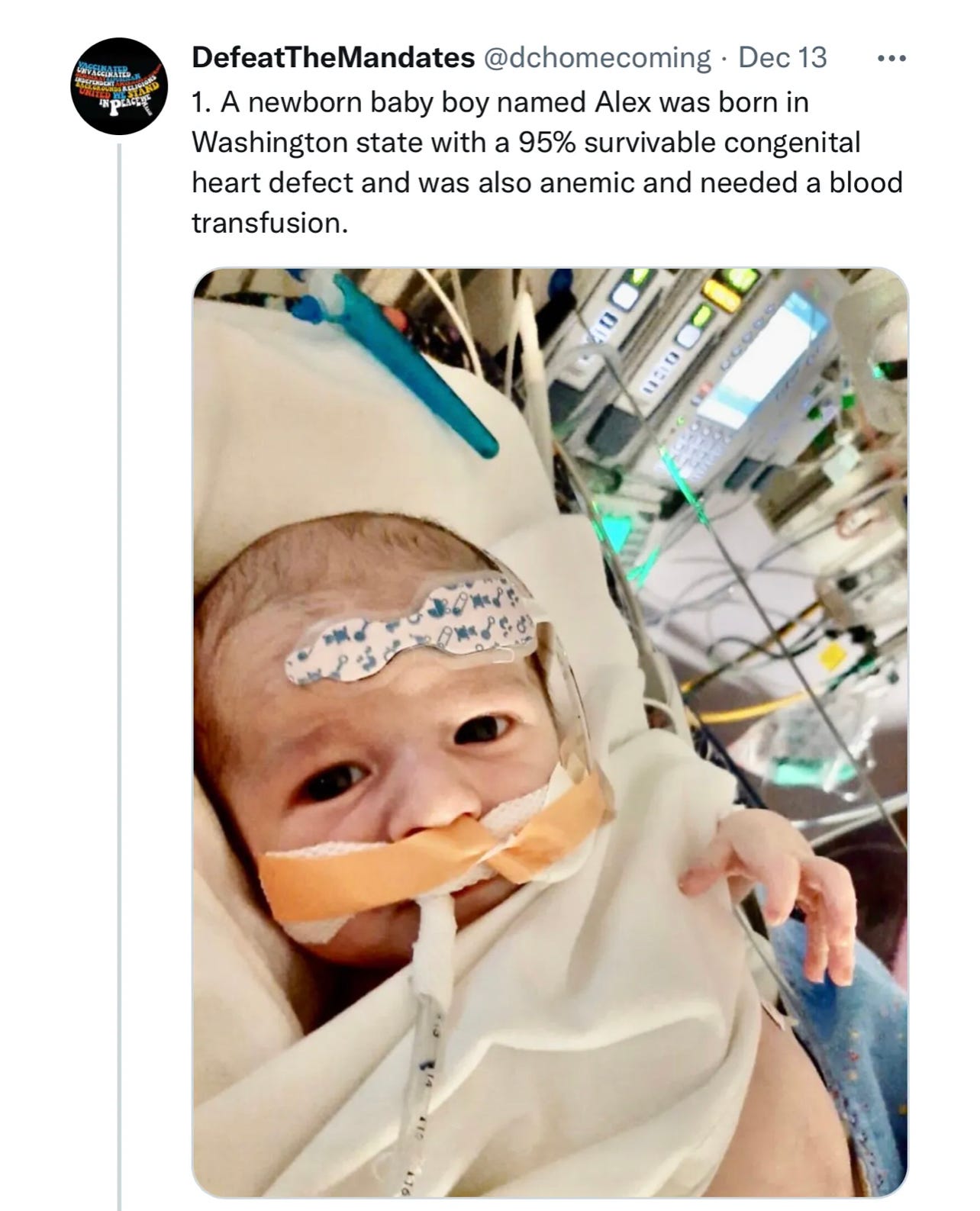
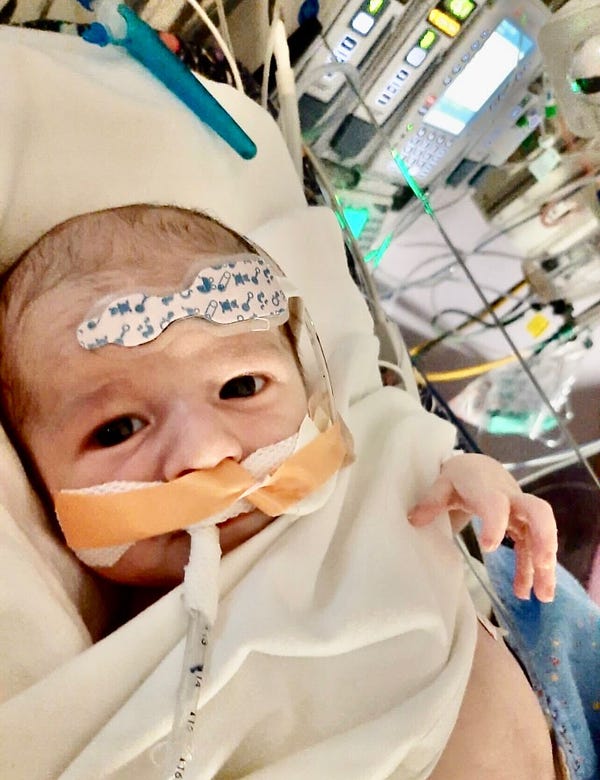
Here is the tragic story (which I wrote an article about last month) of a different baby, Baby Alex, who received blood from the regular supply against his parents wishes, despite unvaccinated blood having been prepared beforehand. The blood was most likely contaminated with spike protein, and the baby passed away from a huge blood clot he quickly developed after the transfusion. He had a 95% survivable heart condition.
Baby Alex was murdered.